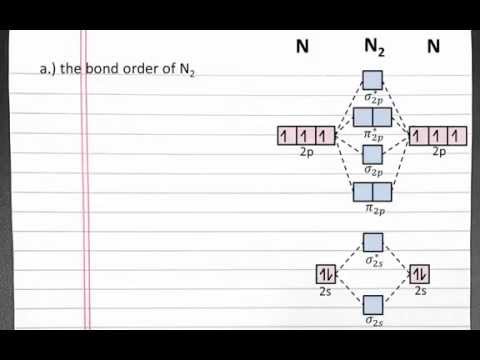
In this example problem, we will use molecular orbital theory to examine the bond order, bond strength, and magnetic properties of different molecules. The problem asks us to determine the bond order of N2, whether it is paramagnetic or diamagnetic, and the order of increasing bond strength for N-N, N-N2, and N2+. First, we will calculate the bond order of N2 by creating a molecular orbital diagram. We start with the orbital diagram for both nitrogen atoms, focusing on the valence electrons. The 2s atomic orbitals of both nitrogen atoms combine to form two molecular orbitals - the bonding and anti-bonding sigma 2s orbitals. The anti-bonding orbital is higher in energy, indicated by an asterisk. Next, we combine the three 2p orbitals of both nitrogen atoms to create six new molecular orbitals - sigma and pi molecular orbitals. The correct order of lowest to highest energy orbitals is determined computationally. To fill our molecular orbitals, we start with the four electrons in the 2s orbitals of both nitrogen atoms. These electrons fill the bonding and anti-bonding sigma 2s orbitals. Then, we use the six electrons from the 2p orbitals of both nitrogen atoms to occupy the lowest available molecular orbitals, which are the pi 2p + sigma 2p orbitals. To calculate the bond order, we subtract the number of electrons in anti-bonding orbitals from the number of electrons in bonding orbitals and divide by 2. In our N2 molecule, we have eight electrons in bonding orbitals and two electrons in anti-bonding orbitals. So, 8 - 2 = 6, and when divided by 2, the bond order of N2 is 3. Moving on to Part B, we determine whether N2 is paramagnetic or diamagnetic. In a diamagnetic molecule, all electrons are paired in orbitals. In a paramagnetic molecule, at least one...
Award-winning PDF software





N244 cc Form: What You Should Know
Maintained. E-filing services. N244(CC) Application Notice. Practice notes. Maintained. E-filing services. N244(CC). Maintained. The Notice was created by Lactates to help you get the right advice to help you to understand the situation where you could make a complaint about your GP or other NHS providers. The Notice does not constitute an application for legal advice.
Online solutions help you to manage your record administration along with raise the efficiency of the workflows. Stick to the fast guide to do N244 Application Notice, steer clear of blunders along with furnish it in a timely manner:
How to complete any N244 Application Notice online: - On the site with all the document, click on Begin immediately along with complete for the editor.
- Use your indications to submit established track record areas.
- Add your own info and speak to data.
- Make sure that you enter correct details and numbers throughout suitable areas.
- Very carefully confirm the content of the form as well as grammar along with punctuational.
- Navigate to Support area when you have questions or perhaps handle our assistance team.
- Place an electronic digital unique in your N244 Application Notice by using Sign Device.
- After the form is fully gone, media Completed.
- Deliver the particular prepared document by way of electronic mail or facsimile, art print it out or perhaps reduce the gadget.
PDF editor permits you to help make changes to your N244 Application Notice from the internet connected gadget, personalize it based on your requirements, indicator this in electronic format and also disperse differently.
Video instructions and help with filling out and completing N244 cc